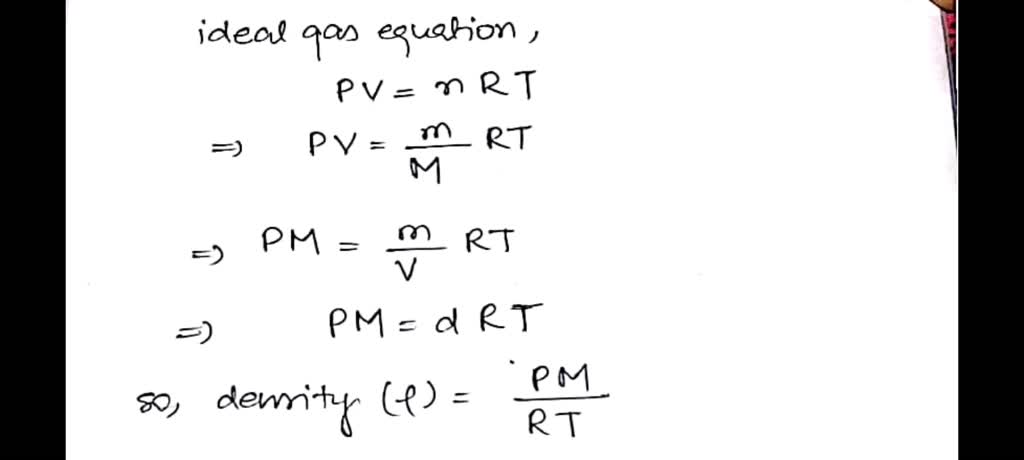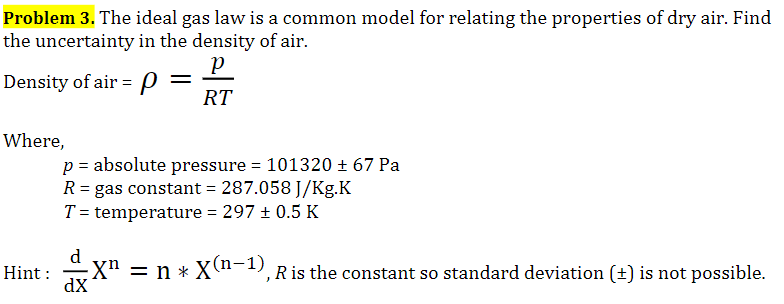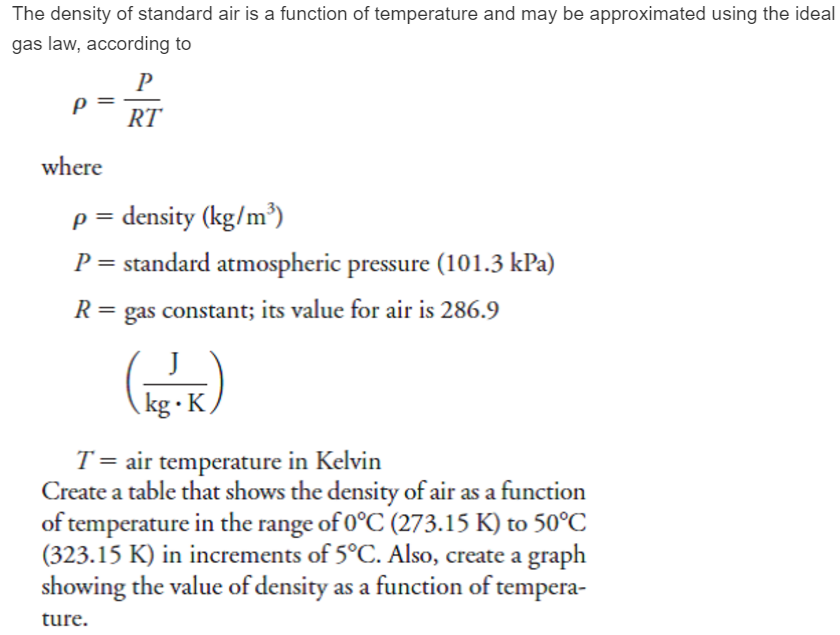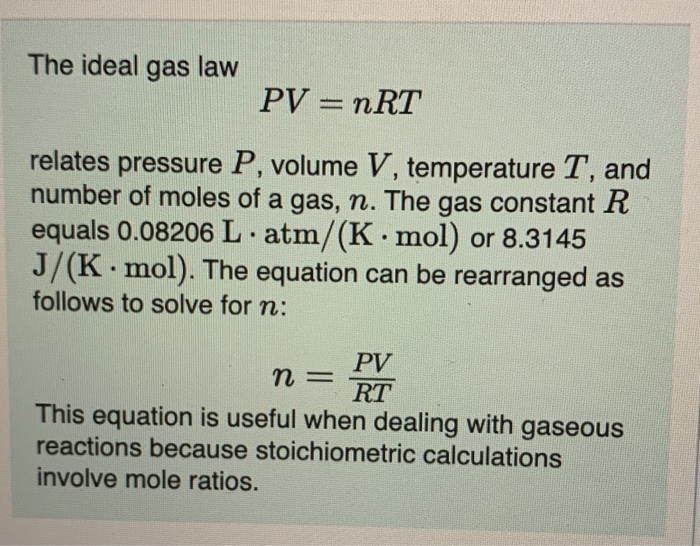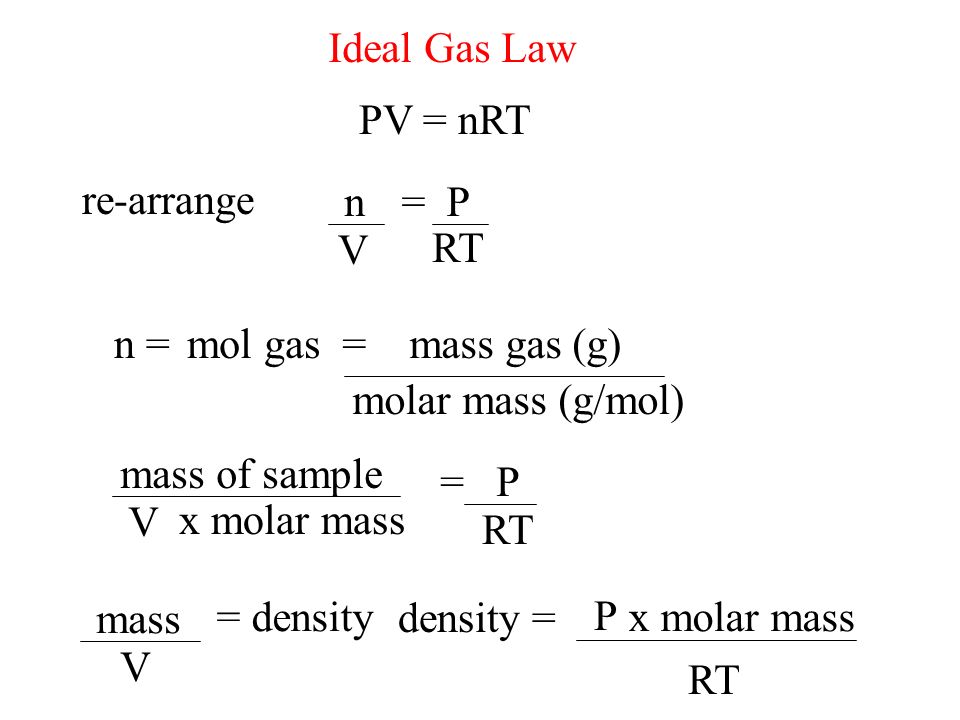
Ideal Gas Law PV = nRT re-arrange n V = P RT n = molar mass (g/mol) mol gas= mass gas (g) mass of sample V x molar mass = P RT =

Ideal Gas Law PV = nRT re-arrange n V = P RT n = molar mass (g/mol) mol gas= mass gas (g) mass of sample V x molar mass = P RT =
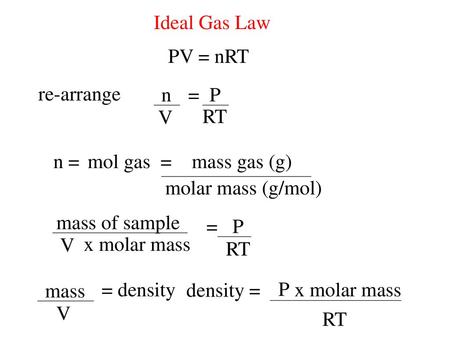
Ideal Gas Law PV = nRT re-arrange n V = P RT n = molar mass (g/mol) mol gas= mass gas (g) mass of sample V x molar mass = P RT =
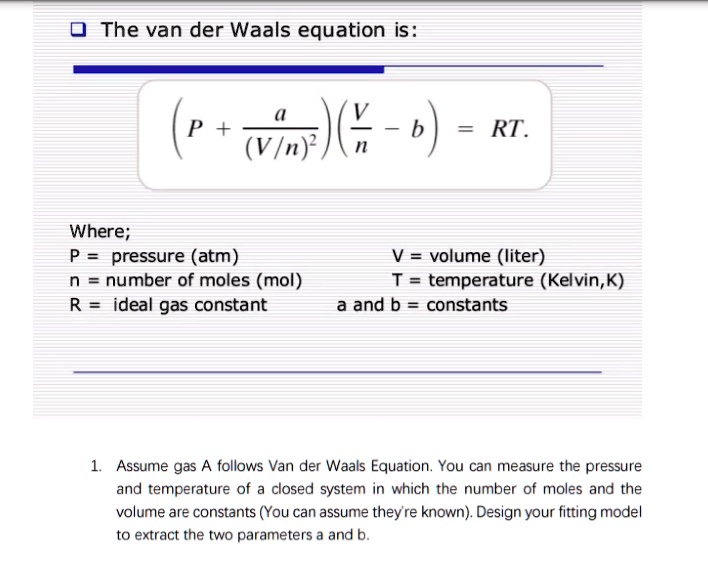
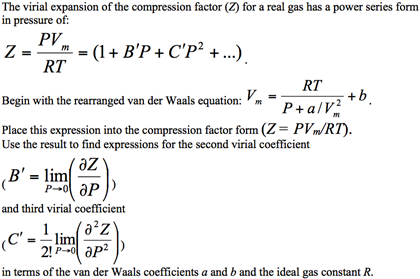
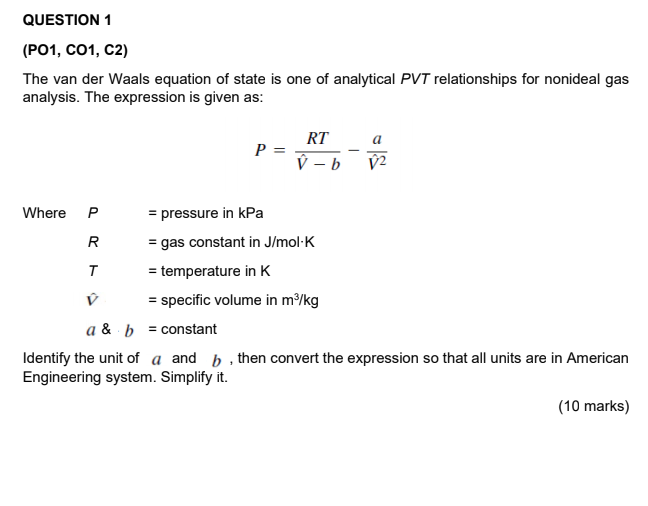
SOLVED: The van der Waals equation is: P + Wo) = b RT. Where; P = pressure (atm) n = number of moles (mol) R = ideal gas constant V = volume (

1 TOPIC 7: GASES Contents Properties of Gases The Simple Gas Laws The Ideal Gas Equation Gases in Chemical Reactions Mixture of Gases Kinetic-Molecular. - ppt download
p+a/v)(v b) =RT, p=pressure,v=volume,R,a,b are constant, T=absolute temperature. find dimension of a/b^2
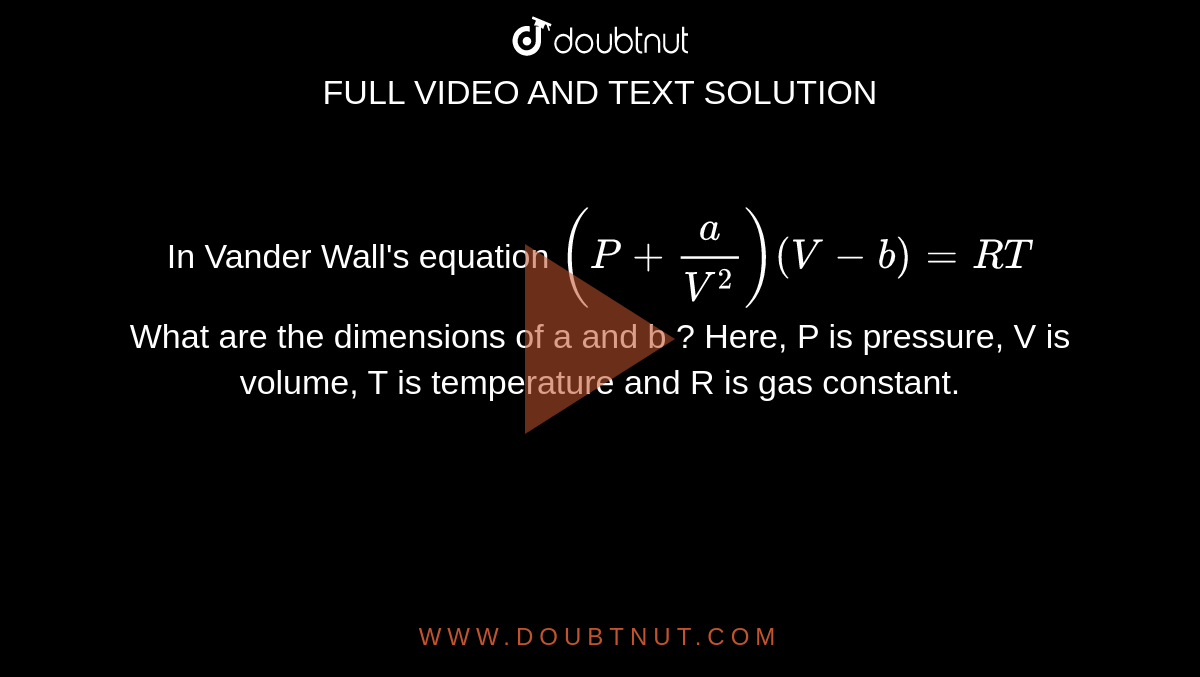
In Vander Wall's equation (P +(a)/(V^2))(V - b) = RT What are the dimensions of a and b ? Here, P is pressure, V is volume, T is temperature and R is

Ideal Gas Law PV = nRT re-arrange n V = P RT n = molar mass (g/mol) mol gas= mass gas (g) mass of sample V x molar mass = P RT =